Keywords: 5-Azacytidine, PCL-Gelatin, Cardiomyocyte differentiation, Electrospinning, Fibrous scaffold, Tissue Engineering
Abstract
India has an alarming rate of growth for cardiovascular diseases. Similar to cancer there is a significant role for epigenetic factors in the increasing prevalence of cardiovascular diseases. Targeting the epigenetic mechanism, namely the DNA methylation processes, histone modifications, and RNA based arrangements are today considered as a potential therapeutic approach to cardiovascular disease management. 5-Azacytidine is an epigenetic treatment drug that is involved in the demethylation of DNA. 5-Azacytidine is an FDA approved drug for myelodysplastic syndrome. However, the usage of 5-Azacytidine for cardiovascular disease has not been found acceptable because of its poor stability in neutral solutions and shorter half-lives which makes it toxic to the cells. A significant breakthrough in the use of 5-azacytidine for cell therapy and tissue engineering of cardiovascular disease treatment has been gained based on its ability to differentiate mesenchymal stem cells to differentiate into cardiomyocytes. This work addresses the further need for a sustained release of this drug, to reduce its toxicity to the stem cells. Electrospun PCL-gelatin fibres that are well aligned to provide a mat-like structure with sufficient porosity for differentiated cells to move forward have been synthesized. The crystalline character, porosity, fibre width, thermal behavior and hydrophilicity of these scaffolds are in tune with those reported in the literature as ideal for cell proliferation and adhesion. FTIR measurements confirm the entrapment of 5-azacytidine on to the scaffold. The adsorption of the drug did not alter the characteristic features of the scaffold. Primary results on cell viability and cell morphology, as well as cardiomyocyte differentiation, have shown that PCL-gelatin scaffolds carrying 5-azacytidine developed in this work could serve as an ideal platform for mesenchymal stem cell differentiation into cardiomyocytes.
Introduction
Development, restoration, and maintenance are a critical aspect for improvement of tissue function when substituted with engineered tissue. Template provided for tissue development is to be resorbed in a sustained manner. The use of materials for tissue replacement and repair can be traced back to thirty thousand years back. With the increasing demand for implants every year, an ideal biomimetic material serves as a platform for cell growth, signaling, interaction repair and for the preservation of structure. In particular, porous structure and porosity of scaffold are essential in drug delivery, cell growth and cell adhesion. Systematic and controlled release of drug for effective treatment and reduced toxicity has attracted researches, especially in nanobiotechnology applications to prepare nanomaterials with porous structures. The systematic approach of drug release involves two steps, namely drug released from the surface and entrapped drug released from the scaffold in three different states. The progress of cardiac tissue engineering in areas such as implantation of myocardial tissue in infarcted rat hearts, in vitro tissue generation for myocardial repair, developing 3D constructs based on collagen gels, and others have been of a very high order. Both cell-scaffold and cell-sheet engineering approaches for cardiac tissue engineering have proven to be effective. Though both these approaches have been proven effective in some contexts developing functional bioengineered cardiac tissues is still challenging. With cardiac tissue engineering gaining prominence for the treatment of cardiovascular diseases, the focus on reducing the concentration of 5-azacytidine to induce cell differentiation has gained fame.
Yu and colleagues reported that culturing mesenchymal stem cells with collagen nano-molecules as scaffolds lead to alterations in differentiation induced with 5-azacytidine. Ex vivo pretreatment of adipose-derived stem cells using 5-azacytidine and use of suitable patterned nanofibrous scaffolds lead to cardiomyogenic differentiation and good functional effects. Interestingly, the patterned nanofibrous scaffolds are generated via electrospinning technique. Russo and colleagues have also suggested that 3D porous scaffolds, preferably tissue-specific extracellular matrix derived, provided for significant enhancement of cardiomyogenic differentiation with 5-azacytidine as inducing agent. Improved human mesenchymal stem cell differentiation has been reported using carbon nanotube-containing electrospun scaffolds of poly epsilon-caprolactone in the presence of 5-azacytidine.
Therapeutic drugs are incorporated into polymeric solutions by several ways including electrospinning, with efficiency enhanced by matching drug and polymer charges. The drug encapsulation efficiency of gelatin is one among the best. Release of drug is based on the distribution of the drug in the electrospun fibres and the morphology of the fibres. For maximum efficiency lipophilic and hydrophilic drugs need to be entrapped into polymers that are lipophilic and hydrophilic. For slow release, combining of hydrophilic-hydrophobic polymers via various polymer combinations is suggested. Gelatin has been reported amongst such polymers that could increase the drug loading efficiency and reduces the burst release rate.
By precipitation casting, microporous scaffolds of PCL-gelatin carrying lactose is generated. The size of gelatin particles influenced the lactose release, with particles in size range of 90 to 250 micrometers displaying 90% drug release over 21 days as against 80% over three days for PCL alone scaffold.
Advancement in human stem cell biology, tissue engineering, nanomedicine, and material science has led to the clinical application of engineered cardiac tissues. Standardization of myocyte production protocols, developing scaffolds that can release the cell differentiation-inducing agents such as 5-aza in a sustained manner, methods for in vitro vascularization of 3D constructs are required for successful preclinical trials.
Based on the identified lacunae in the literature, this work aims to develop a scaffold that could provide an ideal platform for mesenchymal stem cell differentiation and proliferation while reducing the toxicity of the inducing agent through a systematically controlled release.
Materials and Methods
Materials
5-Azacytidine was purchased from Alfa-Aesar. Dulbecco’s modified eagle medium, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, Phosphate buffer saline, Coomassie blue G250, Antibiotic, Antimycotic, PCL and Primers were purchased from Sigma-Aldrich. Fetal bovine serum, Dimethyl sulfoxide, Trypsin-EDTA, Bovine Serum albumin, Carbinol, PBS powder, Sodium Hydroxide, EDTA, Gelatin, phosphoric acid, DEPC were obtained from Hi-Media. C3H10 mouse Mesenchymal stem cells were the kind gift from SRM University. Chloroform, Acetic acid, Agarose, DMSO, Isopropanol, Tris HCl, Bromophenol blue from SRL. dNTP, Oligo dT, RT enzyme and PCR master mix from Takara, Japan. Other reagents used were of analytical grade. Milli Q water was used to dissolve water-soluble chemicals.
Fabrication of PCL-Gelatin Scaffold by Electrospinning Method
The scaffolds were fabricated as described by earlier methods. Solution A: 7% PCL solution was prepared by dissolving PCL in 3:1 volume to volume ratio of chloroform and methanol solvent, followed by mixing using a magnetic stirrer for 2 hours at room temperature. Solution B: 3% solution of gelatin was prepared in 80% glacial acetic acid by mixing thoroughly using magnetic stirrer for 3 hours at room temperature. Solution A and Solution B were mixed in 7:3 ratio and homogenized by stirring for 24 hours. The homogeneous solution obtained after 24 hours was employed for fabrication of electrospun scaffold. For drug entrapped scaffolds, 5-Azacytidine at 100 micromolar and 500 micromolar was added during stirring of solution A and B. Drug loading to polymer solutions and entrapment of the drug in the scaffold is also described in earlier methods. The PCL-gelation solution with or without 5-Aza was taken in a 5 milliliter plastic syringe with a needle. The electrospinning conditions were voltage of 10 kilovolts, drum speed at 750 rpm and flow rate at 0.6 milliliter per hour and distance of 8 centimeters was maintained between needle and collector. The fibers were collected on a 30 by 21 centimeter aluminum sheet. For convenience, Polycaprolactone gelatin, Polycaprolactone gelatin with 100 micromolar concentration of 5-Azacytidine, Polycaprolactone gelatin with 500 micromolar concentration of 5-Azacytidine are abbreviated as PCL-Gelatin, PCL-Gelatin-Aza1 and PCL-Gelatin-Aza2 throughout this study.
Characterization of Scaffold
Surface morphology analysis using SEM was carried out from 2 square centimeters cut portion of the PCL-Gelatin, at an accelerating voltage of 10 kilovolts. Scaffold was viewed at various magnifications. Fiber diameters were calculated using Image J software online tool. Porosity of the scaffold was also measured using Image J software and percentage calculated.
The thermal stability of scaffolds was determined by DSC over a range of 25 to 300 degrees Celsius at a heating rate of 5 degrees Celsius per minute in a nitrogen atmosphere.
To understand the crystalline features powder XRD of PCL-Gelatin, PCL-Gelatin-Aza1 and PCL-Gelatin-Aza2 was obtained using a Miniflux II, Rigaku diffractometer with a CuKα radiation at wavelength equals 0.1548 nanometers, operating in a scanning range of 10 to 80 degrees, with a scan speed of 1 degree per minute was employed.
Infrared spectra of PCL-Gelatin, PCL-Gelatin-Aza1 and PCL-Gelatin-Aza2 scaffolds were taken in the range of 650 to 4000 per centimeter. IR spectra were recorded on an ABB MB 3000 FTIR spectrophotometer.
Swelling Study
The swelling behavior of scaffolds was studied to understand the amount of medium and nutrients that can enter the porous fibrous scaffolds. The scaffold was cut into equal weights of 1 square centimeter and immersed in PBS at pH 7.4 and weight change after 24 hours noted. For this, initial weight of scaffold was taken, followed by immersion in PBS. After defined time interval, the scaffolds were taken out carefully and immersed in water to remove any excess salts and dried using filter paper and dry weight is measured. The percentage of medium uptake in scaffolds was calculated using the formula given below: final weight minus initial weight divided by initial weight multiplied by 100%.
Degradation Study
Studies were carried out to determine ability of scaffolds to degrade over a period of time. The scaffolds were cut into equal weights and carefully immersed in PBS at pH 7.4. The PBS solution was changed every 2 days to determine the activity of scaffold degradation. Initial weight of scaffold W0 was measured and after different time period intervals the scaffolds taken out carefully and dried completely and the dry weight of scaffold is measured W1. The degradation rates were calculated with formula as given below: percentage equals W0 minus W1 divided by W0 multiplied by 100%.
Protein Adsorption Study
Studies were carried out to determine the proteins adsorbed in scaffold. Standard protein adsorptions were estimated by Bradford assay. To evaluate the adsorption of proteins in scaffolds, the scaffolds were cut into equal weights and incubated with two different proteins BSA and FBS. After 3 hours and after 2 days of time period interval amount of adsorbed proteins were calculated.
Cell Culture Studies
1X DMEM medium powder along with 3.75 grams of sodium bicarbonate was dissolved in 90 milliliters of autoclaved milli-Q water was taken and the pH was adjusted to 7.5. 10 milliliters of Fetal bovine serum was added along with 1 milliliter of antibiotic or antimycotic solution to prepare 10% FBS containing medium.
Proliferation and differentiation study using 5-azacytidine treatment was carried out on C3H10 Mouse Mesenchymal stem cells. Mesenchymal stem cells with 5-Azacytidine: The mesenchymal stem cells were supplemented with enriched DMEM for 24 hours in 6 well culture plates and after 24 hours of seeding, the medium was completely removed and fresh medium was supplemented. The cells were treated with 10 micromolar and 20 micromolar 5-Azacytidine. After 48 hours of incubation, the cells were washed with PBS and fresh medium without the 5-Azacytidine was added and placed in the CO2 incubator for cardiomyocyte differentiation study. The medium was changed every 2 days until the study was completed after 15 days of treatment. Mesenchymal stem cells without 5-Azacytidine: The mesenchymal stem cells were seeded in 6 well culture plates without 5-azacytidine treatment and supplemented with DMEM containing 10% FBS for proliferation. After proper propagation and proliferation, the cells were washed with PBS and medium changed every 2 days until the study is completed. Mesenchymal stem cells with PCL-Gelatin scaffold: The mesenchymal stem cells were grown on sterile electrospun 1.5 centimeter PCL-Gelatin scaffold and supplemented with fresh enriched DMEM medium containing 10% FBS in 6 well culture plates for proliferation. After supplementation, the cells were incubated and after every 2 days the medium was changed until the study was completed. Mesenchymal stem cells with 100 micromolar of 5-azacytidine in PCL-Gel scaffold: The mesenchymal stem cells were grown on sterile electrospun 1.5 centimeter PCL-Gelatin-Aza1 scaffold in 6 well culture plates and supplemented with fresh DMEM medium with 10% FBS for proliferation and differentiation of cells. After the supplementation, the cells were incubated and after every two days the medium was changed to ensure no toxicity to cells from scaffolds and it was repeated until the study was completed. Mesenchymal stem cells with 500 micromolar of 5-azacytidine in PCL-Gel scaffold: The mesenchymal stem cells were grown on sterile electrospun 1.5 centimeter PCL-Gelatin-Aza2 scaffold in 6 well culture plates and supplemented with fresh DMEM medium with 10% FBS for proliferation and differentiation of cells. After the supplementation, the cells were incubated and after every two days the medium was changed to ensure no toxicity to cells from scaffolds and the change of medium were repeated until the study was completed.
Cytocompatibility by MTT Assay
Prior to cell seeding, scaffolds were sterilized thoroughly and allowed to swell. A 96 well plate was taken and scaffolds added to appropriate wells. 2 multiplied by 10 to the power of 3 C3H10 mesenchymal cells were seeded and supplemented with medium. After 48 hours of incubating the plates in CO2 incubator, the plates were taken out and medium was removed. The cells were washed with PBS buffer thrice to remove non-adherent cells. 100 microliters of MTT reagent was added and the plate was incubated in dark at 37 degrees Celsius for 4 hours. The reagent was removed and 50 microliters of DMSO was added. A purple color formation as a result of viable cells was monitored at 570 nanometers using microplate reader. Absorbance was compare with the control cells and plotted.
Morphological Changes of Cells Using Optical Microscope
The morphology of the C3H10 cells seeded for attachment and proliferation into fibroblast structure were monitored. The cells seeded for treatment with different concentrations of 5-azacytidine and PCL-Gelatin, PCL-Gelatin-Aza1, PCL-Gelatin-Aza2 was monitored at regular intervals and imaged for the differentiation of stem cells to cardiac cells.
Characterization of Cells at Molecular Level by RT-PCR
Harvesting Cells: The treated cells and control cells containing medium were discarded and washed with sterile 1 milliliter PBS. Then the PBS was discarded and again 1 milliliter of PBS was added and with use of scrapper the cells were gently scrapped all over the wells gently and collected into small microfuge tubes and centrifuged at 1000 rpm for 5 minutes. Then the supernatant was discarded and the pellets were stored at minus 20 degrees Celsius for PCR analysis.
To isolate RNA, the cell pellets were lysed by the addition of 1 milliliter trizol to each tube and pipetted out gently. The lysed cells were stored at 4 degrees Celsius for 5 minutes. Then 0.2 milliliters of chloroform was taken in each tube containing lysed cells and vortexed for 15 seconds and kept for 5 minutes on ice at 4 degrees Celsius. Cells were then centrifuged at 12,000 rpm for 15 minutes at temperature of 4 degrees Celsius. After centrifugation, 3 visible layers were formed. The upper aqueous layer containing RNA was carefully removed and transferred to a fresh sterile microfuge tube and equal volume of isopropanol was added and kept for 10 minutes incubation at 4 degrees Celsius. After incubation, cells were subjected for centrifugation at 12,000 rpm for 10 minutes. The RNA pellets formed were washed twice with 75% ethanol at centrifugation speed of 7,500 rpm for 5 minutes. The RNA pellets were then taken and by gentle pipetting mixed with 50 microliters of sterile DEPC water, followed by 10 minutes incubation for solubilization. RNA thus obtained was spectrophotometrically quantified at absorbance of 260 nanometers. RNA was also subjected for RT-PCR. cDNA was synthesized. For amplification of gene, the following primers were used: Cardiac Troponin T Forward: TCCCCGATGGAGAGAGAGTG and Reverse: AACGAGCTCCTCCTCCTCTT.
0.25% Agarose was added to 25 milliliters 1X TAE buffer and was then melted in a heating mantle and 1 microliter of 1% EtBr were added, mixed evenly and cooled to room temperature. It was then poured into a gel-casting platform with comb inserted for the wells. The gel was then allowed to solidify and after 20 minutes, the comb was removed gently without disturbing wells from the gel. The platform was then immersed completely in the tank filled with electrophoresis TAE buffer. 5 microliters of PCR products from each reaction tube was mixed with 2 microliters of gel loading dye Bromophenol blue and loaded to each well. The power supply was turned on and the current adjusted to 80 volts, for 2.5 hours, and then the resolved cDNA fragments in the gel were visualized and documented.
Statistical Analysis
All the experiments were done in triplicate and data are presented as means plus or minus standard deviation.
Results
Characterization of Scaffolds
SEM micrographs at magnification ranging from 118X to 16500X provide information about the microstructure of scaffold. It can be seen that the scaffold is made up of fibrous matrix with fiber diameter in the nanometer range. Such fibrous structure can help cell adhesion, migration, and differentiation. Percentage porosity of the fibrous mat was determined from the SEM image and employing the Image J software. For depiction purpose, the SEM image was edited using Adobe Photoshop, where the fibres are presented as blue-white strands and the pores as black background. The porosity was 24% at 500 micromolar 5-Aza entrapment, as against 16% for 100 micromolar 5-Aza entrapment and 12.8% for PCL-Gelatin scaffolds without drug.
To understand changes in the thermal properties and the crystallinity of scaffold, the polymer scaffolds were subjected to thermal analysis. It can be seen that the endothermic peak of scaffolds were at 63 degrees Celsius, 56.5 degrees Celsius and 60 degrees Celsius for PCL-Gelatin, PCL-Gelatin-Aza1 and PCL-Gelatin-Aza2, respectively. The endothermic peak was intense and narrow for PCL-Gelatin when compared to drug entrapped scaffolds. X-ray diffraction patterns of the scaffold indicate a crystalline nature of fibres. The increase in intensity at 22.08 can be seen in the following order: PCL-Gelatin greater than PCL-Gelatin-Aza2 greater than PCL-Gelatin-Aza1. IR analysis of PCL-Gelatin scaffold and drug entrapped gelatin scaffold shows the characteristic bands of PCL-Gelatin scaffold. The shift in the vibrational mode of bands is suggestive of molecular interaction of 5-Azacytidine to PCL-Gelatin. The shift in the vibrational modes at these frequencies indicates the involvement of these to azacytidine which could be via amide stretch as well as hydrogen bonding.
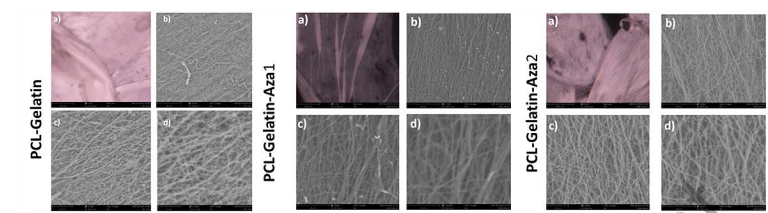
Figure 1. Electrospun blend SEM images of PCL-Gelatin, PCL-Gelatin-Aza1, and PCL-Gelatin-Aza2 blends for electrospinning at different magnifications: a) 118×, b) 2250×, c) 5500×, and d) 12500×.
Biological Characterization of Scaffolds
Swelling behavior of scaffolds were monitored in PBS solution. An increase in swelling can result in the increase in contact of solvent to the drug, which in turn can facilitate release of the drug. Drug loaded scaffolds showed an increase in swelling behavior which could be due to the increase porosity. To understand the degradation process, the scaffolds were immersed in PBS and monitored at three time intervals of one week, two weeks and one month. It is evident that as the time period increased, the scaffolds degraded gradually. For PCL-Gelatin scaffolds, the percentage degradation was 20.72, 27.9 and 36.7 at 1, 2 and 3 week periods respectively. The values were 8.71, 17.64 and 23.79 for PCL-Gelatin-Aza1 scaffolds, and 15.49, 35.2 and 42.53 for PCL-Gelatin-Aza2 scaffolds respectively. Protein adsorption of scaffolds at different time intervals shows that at three hours of scaffold incubation, the adsorption of protein was more for PCL-Gelatin scaffolds at 90 micrograms. For drug entrapped PCL-Gelatin, PCL-Gelatin-Aza1, PCL-Gelatin-Aza2, the adsorption was found to be 61.6 and 52.3 respectively. When scaffold was immersed in PBS for few hours and then immersed in BSA containing solution for 48 hours, there was an increase in adsorption of protein at 392, 395 and 370 micrograms for PCL-Gelatin, PCL-Gelatin-Aza1, PCL-Gelatin-Aza2 respectively.
Interaction of Scaffolds with Cells
Cellular morphology was observed for 5-Azacytidine treated C3H10 mesenchymal stem cell cells when compared to control cells which showed stick like cells that are connected to form myotube like structures. The expression of cardiac troponin specific marker for in vitro cardiomyogenic differentiation of stem cells can be seen only in azacytidine induced cells. The increase in expression of troponin could be due to increase in the concentration of 5-azacytidine from 10 to 20 micromolar. Preliminary studies on the cell viability of C3H10 mesenchymal stem cells treated with 5-Azacytidine entrapped in different concentrations has shown promising results as indicated by the absorbance in the graph which is a measure of metabolic activity of cells. There was no statistical difference in groups: PCL-Gelatin, PCL-Gelatin-Aza1, PCL-Gelatin-Aza2 when compared to control by Students t-test.
Cells were incubated with 0.5 centimeters of the scaffold which is equivalent of 0.002 milligrams Aza1 and 0.012 milligrams of Aza2 and the viability of cells in MTT assessed after 48 hours. Cellular morphology of 5-azacytidine entrapped PCL-Gelatin treated C3H10 mesenchymal stem cell cells when compared to control cells that showed the stick like cells. Cardiac Troponin T analysis of the cDNA and agarose gel electrophoresis suggests that the 5-Azacytidine entrapped PCL-Gelatin scaffolds of different concentrations successfully differentiated mesenchymal stem cells into cardiomyocytes. The intensity of expression was analyzed using Image J software as seen in the graph. These findings suggest that 5-Azacytidine could be incorporated into porous scaffold for sustained release of drug that can reduce toxicity to cells and can increase the rate of differentiation of cells.
Discussion
One of the versatile techniques to produce fabricated scaffolds for tissue engineering and potential drug delivery applications is electrospinning. Earlier reports have suggested the most effective cellular interactions of drug incorporated in the polymer matrix. The present study was attempted to include 5-Azacytidine with the potential of differentiating stem cells to cardiomyocytes. The main challenges of using this drug are to find an effective method by which the cells can be differentiated at a very high rate with low toxicity. By tuning various parameters like voltage at 10 kilovolts, a flow rate of the polymer at 0.6 milliliters per hour, tip to collector 8 centimeters distance and 750 rpm drum speed a fibrous sheet of 30 by 21 centimeters length PCL-Gelatin, PCL-Gelatin-Aza1 and PCL-Gelatin-Aza2 scaffold was fabricated by the electrospinning method. As observed from SEM images, the PCL-gelatin scaffolds with varying loads of 5-azacytidine presented exciting results. The fibre width in all cases varied from 60 to 1000 nanometers. There was no noticeable influence of 5-azacytidine when the concentration was 10 micromolar. However, the width of the fibres tends to increase as the 5-aza concentration was increased further. A histogram of the fibre width prepared through analysis of over 100 fibres using ImageJ software and plotting the same using online tools is presented in the supplementary information.
Electrospinning technique resulted in long straight fibres oriented in predominantly two directions, thus resulting in the well-knit mat-like structure. Fibrous scaffolds are better for cell attachment than solid film cast methods. The observed porosity is in tune with earlier reported values, where strong cell attachment and proliferation have been reported. It is reported that pore size influences the cell process, where nano pore size membranes are useful in formation of collagen fibres and ECM and macropores for cell seeding, distribution and migration. Further, in the case of osteoblasts in 48 hour cultures, scaffolds of 120 micrometers pores had better cell adhesion and proliferation and after 7 days of culture, with 325 micrometers pores cell migration was better. The observations in this study are in tune with the reported literature.
The DSC measurements were carried out to understand the thermal nature of the scaffolds by heating at higher temperatures, which provides information about the exothermic and endothermic reactions. The results show the endothermic peak for PCL-Gelatin at 63 degrees Celsius also indicating a sharp narrow peak of crystallinity. PCL-Gelatin-Aza1 shows a peak transition temperature of 56.5 degrees Celsius with lesser intensity of peak suggesting the decrease in crystallinity when compared to PCL-Gelatin. For PCL-Gelatin-Aza2, figure shows a narrow peak and a transition temperature of 60 degrees Celsius due to higher concentration of drug incorporation in scaffold, when compared with PCL-Gelatin-Aza1. Results of difference in the endothermic peaks suggest the entrapment of 5-Azacytidine in PCL-Gelatin scaffolds with different transition temperature for different concentration. It is known that there is a synergic effect between the entrapped drug and scaffold crosslinking. The change in the transition temperature is on entrapment of the drug is attributed to the interaction of the polymer chain with the surface of the drug particles, which in turn changes the chain kinetics.
The XRD patterns of PCL-Gelatin scaffold shows that the PCL-Gelatin scaffolds shows a very sharp peak at intensity 490 at 2θ equals 22.08 exhibiting a fine crystalline property of scaffold. The diffractogram of PCL-Gelatin-Aza1 shows a characteristic peak at 2θ equals 22.08 with intensity at 300 that could be as a result of drug incorporation and the beaded formation. The diffractogram of PCL-Gelatin-Aza2 showed a sharp peak at 2θ equals 22.08 with an intensity at 390, also giving crystalline property. The greater intensity at 390 for PCL-Gelatin Aza2 than PCL-Gelatin-Aza1 is possibly due to absence of bead formation and incorporation of drug. From the results it can be concluded that the decrease in intensity of PCL peaks could be reflected as an incorporation of drug to the scaffold that could have decreased the crystallinity of the scaffolds. This decrease in intensity of PCL peaks is attributed to the coordinate property of the gelatin, similar to reported literature on PCL-Chitosan.
FTIR analysis was carried out to understand the interaction of 5-Azacytidine in PCL-Gelatin matrix. The shift in the CH2 stretching, carbonyl stretching, C-O-C stretching and N-H bending is shown. Earlier reports have suggested the mode of interaction of PCL to Gelatin to be via the CO-NH interaction. The IR spectral results suggests the interaction of 5-Azacytidine at the back bone of CH2 stretching.
For permeability of biomolecules, an important parameter of consideration is the ability of the fabricated scaffold to imbibe water content. Increase in surface to volume ratio of scaffold will result in increase in the infiltration of cells leading to cell adhesion. Moreover, the porous nature of the scaffold can facilitate appropriate cell seeding with proper exchange of nutrients to the cells. The results show the swelling property of scaffold soaked in PBS for 24 hours. The results show that an increase in pore size of the PCL-Gelatin-Aza1 and PCL-Gelatin-Aza2 scaffolds has resulted in more uptake potential than PCL-Gel scaffold.
Drug release into biological fluids from scaffolds is important to understand the sustained drug release properties. The drug release in medium from scaffolds is accompanied by the swelling of matrices, which helps diffusion of solutes via pores of scaffolds, while maintaining the structural integrity by uptake of nutrients. The temperature and pH of the medium can also play an important role in drug release from scaffolds. Recently, the use of biopolymers has gained interest in tissue engineering due to its biodegradability. Biodegradability of a scaffold can result in the sustained release of drug into the microenvironment that can promote cell differentiation and regeneration. In this study, PCL-Gelatin scaffolds soaked in PBS for 1 week, 2 weeks and one month resulted in gradual degradation of scaffold at 36%, probably due to the retaining of the RGD sequence by Gelatin that can promote cell adhesion, differentiation and degradation. The PCL-Gelatin-Aza1 resulted in a slower degradation rate of 23%. PCL-Gelatin-Aza2, on the other hand had a higher degradation rate of 43%. The decrease in degradation of PCL-Gelatin in the presence of Aza1 could be attributed to the ability of Aza1 to provide structural integrity to the structure, while with increasing concentration of azacytidine Aza2, the structure becomes brittle and degrades faster. These results are in tune with earlier observation with respect to the loading of graphene oxide on chitosan-gelatin scaffolds. Thus the results depicts that slow degradation rate up to one month will helps proper cell regeneration of organs as the diffusion of essential nutrients would be maintained, with concomitant degradation of excess wastes from the organ. The surface morphology of fibrous scaffold is highly depending on protein adsorption which facilitates cell adhesion, cell-cell interaction, proliferation and differentiation.
Thus, the present study was formulated to understand the amount of protein BSA adsorbed in the PCL-Gelatin, PCL-Gelatin-Aza1 and PCL-Gelatin-Aza2 scaffolds after for 3 hours and at 48 hours incubation. The results show that PCL-Gelatin with no drug incorporation has 60 micrograms of proteins adsorbed on scaffolds due the RGD sequence of gelatin. The PCL-Gelatin-Aza1 and PCL-Gelatin-Aza2 has slightly similar proteins adsorbed at 3 hours. After 48 hours, the adsorption potential for PCL-Gelatin increases due the fine porous fibre morphology. Similarly, PCL-Gelatin-Aza1 and PCL-Gelatin-Aza2 also shows increase in protein adsorption, though the increase is not statistically significant. The increase in protein adsorption results in better cell proliferation and regeneration. FBS is one of the essential proteins required during cell culture studies supplemented along with essential medium which promotes for attachment, proliferation.
5-azacytidine is a strong inducer for cardiomyocyte differentiation of mesenchymal stem cells by playing the role by modifying DNA at the gene level and inhibiting the DNA methylation, thus leading to differentiation. 5-azacytidine induction on mesenchymal stem cells leads to the hypomethylation and alters the genes for the cardiac cells formation. At some concentration, 5-azacytidine treatment might also induce toxicity. Toxicity studies carried out by MTT assay for different concentration levels of 5-Azacytidine helps in detection of proliferation or apoptosis. Several studies have reported that the induction of 5-azacytidine treatment directly on the cells containing medium results in toxicity at higher concentration and induces apoptosis. The cell viability is directly related to the increase or decrease in absorbance at 570 nanometers. It can be seen that mesenchymal stem cells grown in the presence of PCL-Gelatin, PCL-Gelatin-Aza1 and PCL-Gelatin-Aza2 did not show any toxicity towards cells after 48 hours incubation, suggesting adherence, viability and proliferation due to slow release of drug towards the cells. Preliminary studies showed promising results that direct incorporation of 5-azacytidine on scaffolds can cause slow release to cells with no toxic effects and any concentration within the range investigated in this study will not cause harm to the cells. Further studies are warranted to understand and compare the toxicity effects of 5-Azacytidine treated to cells directly.
At 7 to 8 days of seeding the cells with or without 5-Azacytidine and PCL-Gelatin with or without 5-Azacytidine, morphological changes were observed. It is evident that the morphology of the cells as compared to control and PCL-Gelatin has changed indicating the differentiation of stem cells to cardiomyocytes. The characteristic features of spindle formation, tubular formation that connects cells and tubular structures can be visualized, which clearly indicates the differentiation of mesenchymal stem cells to cardiomyocytes.
The C3H10 mesenchymal stem cells were used for treatment with 5-azacytidine for cardiomyocyte differentiation. Differentiation of mesenchymal stem cells can be inferred from cardiac troponin T expression an indication of the differentiation of mesenchymal stem cells to cardiomyocytes. PCR technique was used to analyze the gene expression at molecular level to confirm the formation of cardiomyocytes. As an early marker of myogenic formation, the cardiac troponin T plays an important role for formation of contractile muscle and muscle tissues. It is also an important protein required for regulating calcium via activity of ATPase by myofibrilla. The present study confirms the differentiation of cardiomyocytes at mRNA level by the expression of cardiac troponin T when cells are treated at 10 micromolar and 20 micromolar concentration of 5-azacytidine. The bands on treatment with 20 micromolar concentration of 5-azacytidine show greater expression than 10 micromolar concentration resulting that CTnT is highly present due increased concentration of 5-Aza and it is not toxic towards cells.
The incorporation of 5-azacytidine in PCL-Gelatin scaffold at varying concentrations has confirmed the differentiation of cardiomyocytes by the gene expression studies. Cardiac troponin T has been expressed at greater intensity in PCL-Gelatin-Aza2 treated mesenchymal cells. The cardiac troponin T expression was also seen in PCL-Gelatin-Aza1 but not in control and PCL-Gelatin treated cells, indicating the importance of 5-Azacytidine in cardiomyocyte differentiation. These results indicate that slow release of drug from scaffold can reduce toxicity towards cells and thus lead to cardiomyocyte differentiation. The incorporation of drugs on scaffolds is promising as a sustained drug delivery vehicle when implanted in damaged site as cure for regeneration.
Conclusions
Challenges in cardiac tissue engineering can be group as those associated with cells, biomaterial scaffolds and vascularization. This work has successfully addressed to challenges in all the groups. Studies indicate that electrospun fibres of poly epsilon-caprolactum gelatin served as an ideal synthetic polymer biopolymer composite scaffold, where fibres were well aligned into a mat like structure having sufficient porosity for transport of the differentiated cells to the surface. Adsorption of 5-azacytidine a cell differentiation-inducing agent on the scaffold did not significantly alter the properties of the scaffold, though fibre width was slightly shifted to the higher size in the histogram. The scaffolds were crystalline and changes in crystallinity were observable on interaction with 5-azacytidine. Noticeable shift in the IR bands of 5-azacytidine was noticed upon adsorption on to the scaffold, thus providing a clear evidence for its interaction with the scaffold. Hydrophilicity required for cell scaffold interaction was noticeable from the increase in weight of the scaffold on immersion in buffer. Porosity of the scaffolds aided their swelling as well. A 40% degradation of scaffold over a period of one month in PBS buffer and in 24 hours under accelerated conditions is a clear proof of the biodegradability of the scaffold. Thermal properties and protein adsorption studies showed promising results and were comparable to those suggested in literature as ideal for cell proliferation, regeneration and adhesion. Results on cell viability and cell morphology as well as cardiomyocyte differentiation have shown that PCL-gelatin scaffolds carrying 5-azacytidine, provides for a sustained release of the drug and thus reduces its toxicity to the cells and differentiates the stem cells to cardiomyocyte. Though further studies are needed to establish the cell differentiation and cell viability of this methodology, the electrospun PCL-gelatin scaffolds developed in this study had the ideal characteristics to serve as a good platform for the proliferation, regeneration and adhesion of stem cells. The significance of this work lies in the ability of the scaffold to serve as a good adsorbent for the cell differentiation inducing agent 5-azacytidine and aid its sustained release leading to low toxicity to the cells.